Catalytic Asymmetric 1,3-Dipolar Cycloaddition
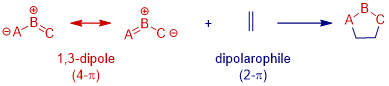
1,3-Dipolar cycloaddition (1,3-DC) reaction is recognized as one of the most powerful tool for the convergent construction of five membered heterocycles. Normally, the reaction take place between 4-p electron 1,3-dipole and 2-p dipolarophile, affording 5-membered heterocycles. Among various dipoles, azomethine ylide is one of the commonly-used one for pyrrolidine synthesis.
.png)
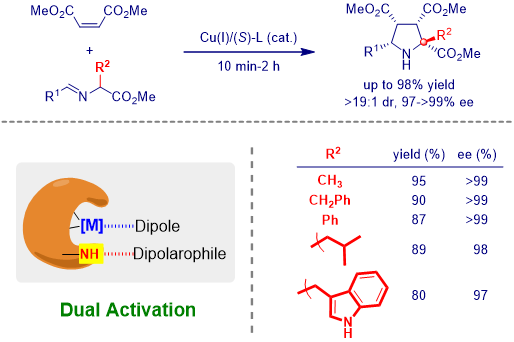
In the last decades, metallated azomethine ylide received much attention in catalytic asymmetric 1,3-dipolar cycloaddition reaction, because its precursor, imino ester, is readily available from simple starting materials. Although, many catalytic systems have been developed for the asymmetric construction of pyrrolidines, there are still some challenges in this area.

For example, most of imino esters are a-unsubstituted imino ester derived from glycinate. Normally, those compounds show much less reactivity and stereoselective control compare with a-unsbustituted imino esters. It may be caused by the unfavored steric hindrance and less acidity of this H atom.
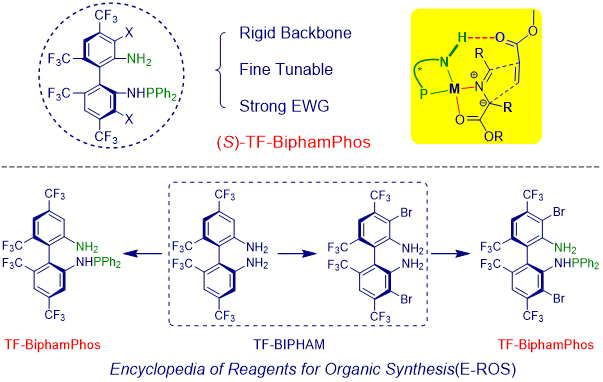
In 2008, our group synthesized a new kind of chiral ligand, named as TF-BiphamPhos, which exhibited excellent catalytic activity in azomethine ylide-involved 1,3-DC reaction, especially for those challenging a-substituted imine esters. Later experimental and computational studies revealed that the H-bonding interaction between the free NH2 group in chiral ligand and the electron-withdrawn group is the key point for enhancing the reactivity and the stereoselectivity control. The application of this methodology was further demonstrated by the construction of the key intermediate of the potent inhibitor of polymerase.
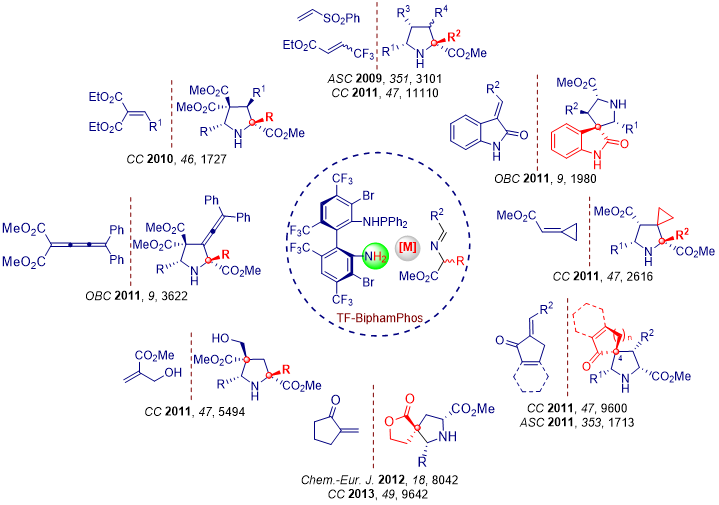
A variety of disubstituted electron deficient alkenes can be employed as dipolarophiles in our systems, various pyrrolidines with quaternary stereocenter were obtained in excellent stereoselectivity. Moreover, some trisubstituted alkenes were also tested and delivering the highly functionalized spiro pyrrolidine-oxindoles, -cyclopropanes and other spiro heterocycles with excellent stereoselective control.
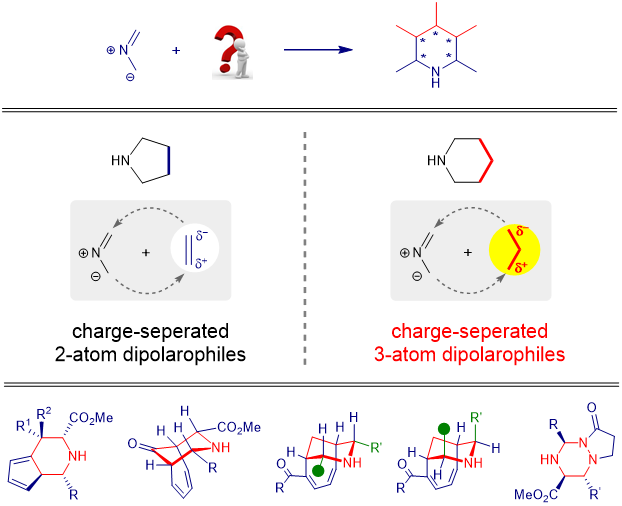
1,3-DC reaction was also called as [3+2] reaction because almost all the dipolarophiles are 2-p components, which provide two atoms in the final 5-member ring adducts. In order to construct six-membered heterocycles, 3-atom synthons must be need, and the positive and negative charge should be present at the two terminal.
We envisioned that some cyclic polyenes maybe suitable for this purpose if the positive or negative charge can be efficiently delocalized through resonance hybridization. Cyclic polyenes, such as fulvenes, tropone and 2-acyl substituted cycloheptatrienes, were used as efficient 6- p dipolarophiles, as the surrogate of charge-separated 3-atom units in 1,3-DC reaction of azomethine ylides, affording enantioenriched six-membered fused and bridged piperidine derivatives. Resonance hybridization provides the driving force for those transformations. Meanwhile, with two different ylides as the reaction partners, we successfully developed the first cross 1,3-DC reaction of two different ylides for the construction of six-membered triazinanes.
 WUHAN University Website
WUHAN University Website
 loading......
loading......